what is true about fecl3 that allows it to form a colored complex with phenols?
2.3F: Visualizing TLC Plates
- Page ID
- 93522
Organic compounds nearly usually appear colorless on the white background of a TLC plate, which means that after running a TLC, chemists often cannot merely encounter where compounds are located. The compounds have to be "visualized" afterward elution, which means to temporarily catechumen them into something visible.
Visualization methods can be either not-destructive (chemical compound is unchanged later on the procedure) or destructive (compound is converted into something new afterwards the process. Viewing a TLC plate under ultraviolet low-cal is non-subversive, while using a chemical stain is subversive.
Visualization Summary
Below is a summary of various visualization techniques, and the functional groups that by and large react with each. A more than detailed discussion of each technique is provided later in this section.
| UV Light: For aromatics + conjugated systems | Iodine: Visualizes ~one-half the time. Strongly reacts with aromatics | p -Anisaldehyde : For many aldehydes, ketones, and alcohols | Vanillin: For many aldehydes, ketones, and alcohols |
 |  |  |  |
| Permanganate: For alkenes, alkynes, or oxidizable groups (aldehydes, alcohols) | Phosphomolybdic Acid (PMA): For alcohols, phenols, alkenes, and many carbonyl compounds | Iron(3) Chloride: For phenols | Bromocresol Green: For acidic compounds |
 |  |  |  |
Table 2.iv: Summary table for TLC visualization methods.
Ultraviolet Absorption
The most common non-destructive visualization method for TLC plates is ultraviolet (UV) calorie-free. A UV lamp can be used to smooth either short-waved \(\left( 254 \: \text{nm} \right)\) or long-waved \(\left( 365 \: \text{nm} \right)\) ultraviolet light on a TLC plate with the touch of a push. Most commercially bought TLC plates contain a fluorescent textile (e.g. zinc sulfide) in the silica or alumina, so the background of the plate will appear light-green when viewing with short-waved UV low-cal. If a compound absorbs \(254 \: \text{nm}\) UV light, it volition appear dark, as the compound prevents the fluorescent cloth from receiving the UV light.
This method is then quick and piece of cake that information technology is often the first visualization method tried. It is most useful for visualizing effluvious compounds and highly conjugated systems, equally these strongly absorb UV. Most other functional groups do not absorb UV light at the wavelengths used and will not appear dark under the UV lamp even though they are however there. It doesn't injure to try UV after performing TLC with all compounds only in example. Since the compounds remain unchanged after viewing with UV light, a further visualization technique tin exist used afterward on the aforementioned plate.
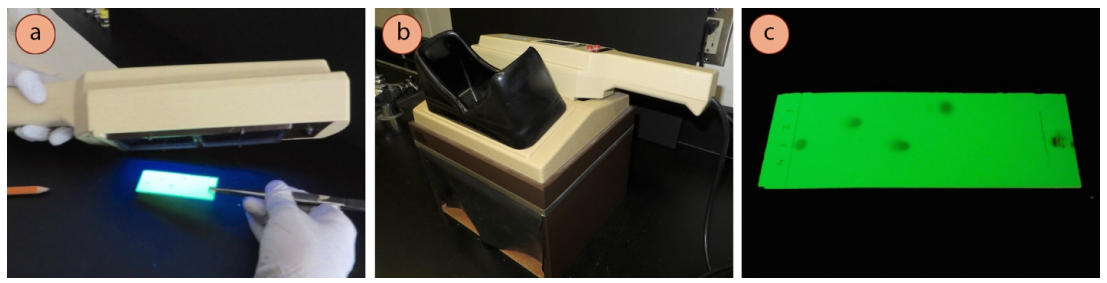
Procedure for UV visualization of TLC plate:
- Use a UV lamp to look at your developed TLC plate by pressing the short-waved button. Depending on what is bachelor at your institution, you lot might tilt the UV lamp at an angle in a darkened area of the room (Figure ii.31a), or you might look at the plate inside a box designed to protect eyes from UV damage (Figure 2.31b).
Safety note: Care should be taken to never look straight at the UV source, and to minimize exposure to eyes.
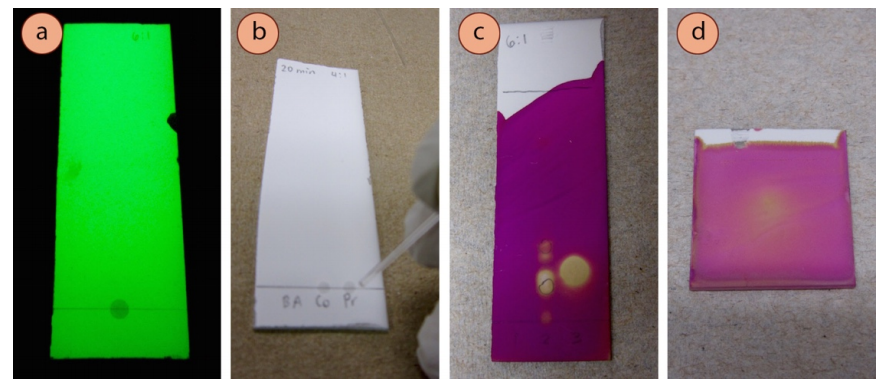
- The plate groundwork will appear light-green under short-waved UV calorie-free, and UV-agile compounds volition appear dark (Figure 2.31c). Apply a pencil to lightly circle spots, equally they will disappear when the UV lite is removed.
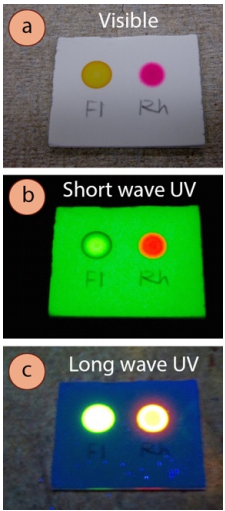
- Some compounds themselves fluoresce (Figure 2.32), appearing a variety of colors when exposed to either brusk- or long-waved UV low-cal (brilliant purple or bluish is the almost common). Record these types of observations in your notebook if you see them, equally they are rare, and are therefore an splendid identification tool. They are most common with highly conjugated compounds.
- The TLC plate can be further visualized with another method if desired (iodine or a chemic stain).
Iodine
A commonly used semi-subversive visualization method is to expose a adult TLC plate to iodine \(\left( \ce{I_2} \right)\) vapor. An "iodine chamber" can be created past calculation a few iodine crystals to a TLC chamber, or by adding a few iodine crystals to a chamber containing a portion of powdered silica or alumina (Effigy 2.33a). When a developed TLC plate is placed in the chamber and capped, the iodine sublimes and reacts with the compounds on the plate, forming yellow-brown spots (Effigy 2.33d). The coloration occurs considering iodine forms colored complexes with many organic compounds. This stain volition work with approximately half the compounds yous may run into.
This method is considered "semi-destructive" because complexation is reversible, and the iodine will eventually evaporate from the TLC plate, leaving the original compound behind. When the coloration fades, it is theoretically possible to utilize another visualization technique on the TLC plate, although information technology's possible the chemical compound may take likewise evaporated by that time.
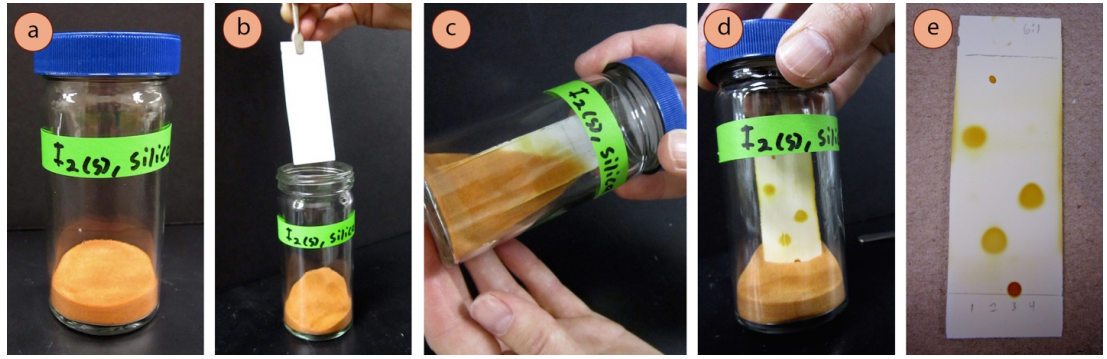
Procedure for visualization of TLC plate with iodine:
- If not already prepared, make an "iodine chamber" (Figure 2.33a): in a smoke hood, identify a few centimeters of powdered silica or alumina in a spiral-capped TLC chamber and add together a few crystals of solid iodine ( rubber annotation: silica and alumina are lung irritants, and iodine vapor is considered an irritant and toxic). A beaker and lookout glass will not work in this context every bit the iodine vapors volition escape. Let the silica or alumina and iodine sit together for a while with periodic swirling, and somewhen the pulverisation volition become orange from adsorbing the iodine vapor.
- In a fume hood, place the adult TLC plate in the iodine sleeping accommodation with forceps (Effigy 2.33b) and close the chapeau. Gently shake the bedroom to bury the TLC plate in the iodine-stained silica or alumina (Figure 2.33c) until the spots become colored (Figure 2.33d). Many spots volition appear yellow-brown almost immediately, and may darken with extended time. For many compounds, it takes less than 10 seconds to develop a plate, but some compounds crave 10 minutes or longer. (Note: alcohols, carboxylic acids, and alkyl halides often practice not stain.)
- Promptly record appropriate observations of the TLC in your notebook, or circle the spots with a pencil, as the colors volition soon fade as the iodine evaporates from the plate. Further visualization may be attempted after the color fades.
Chemical Stains
At that place are a variety of subversive visualization methods that can plow colorless compounds on a TLC plate into colored spots. A plate is either sprayed with or dipped in a reagent that undergoes a chemical reaction with a compound on the TLC plate to convert it into a colored compound, enabling the spot to exist seen with the naked center. Since a chemical reaction is occurring in the process, it is common to gently heat a plate after exposure to the reagent to speed up the reaction, although this may exist unnecessary with some stains. Not every compound can exist visualized with every reagent if they practice not react together, and stains are oftentimes designed to work with only sure functional groups. The specific stain should be chosen based on the presumed structure of the compounds yous want to visualize.
General Staining Process
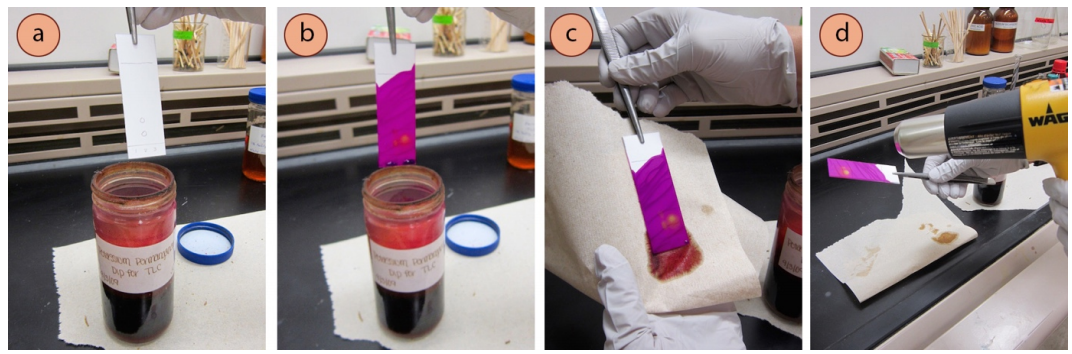
- While wearing gloves and holding the TLC plate with forceps in a fume hood, chop-chop dip the plate into and out of the chemical dip jar so that the stain covers the expanse where the solvent traveled on the plate (Figure ii.34b).
- Let excess liquid drip off the plate for a few seconds, and so wipe the back of the plate with a paper towel (Figure ii.34c).
- Gently rut the plate to develop the spots. Preferably use a rut gun (Effigy two.34d), but a hotplate tin can too be used (Figure two.35, charring is common).
- If using a rut gun, concur the TLC plate with forceps and wave the heat gun back and forth onto the forepart of the plate. The "high" setting can be used at first, with the setting turned to "low" if the plate begins to char. Rubber note: Heat guns are non simple hair dryers, and tin get quite hot. Exist careful to not bear upon the nozzle, and call back that it remains hot for a long time subsequently heating has ceased. The hot nozzles can even mar benchtops, so exist cautious when setting the gun downwardly.
- If using a hot plate, place the TLC plate on the warm surface (set between depression and medium-low, and covered in foil to forbid dip remainder from staining the ceramic surface). Periodically motion the plate around to distribute the estrus.

- A TLC plate cannot be further visualized after using a stain.
p-Anisaldehyde/Vanillin Stains
Generalities
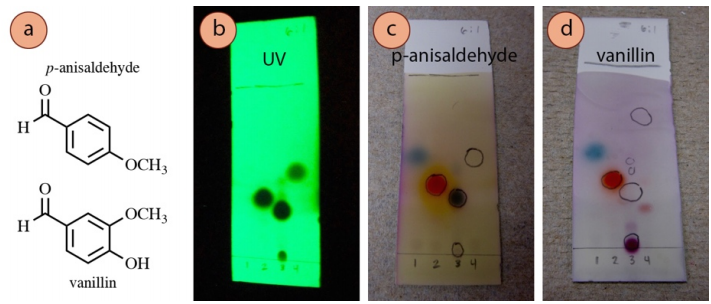
The p-anisaldehyde and vanillin stains are general purpose, and work for many strong and weak nucleophiles (alcohols, amines), and for many aldehydes and ketones. They practise not piece of work on alkenes, aromatics, esters, or carboxylic acids. The TLC plates demand to be mildly heated, and will develop a light pinkish to dark pink background.
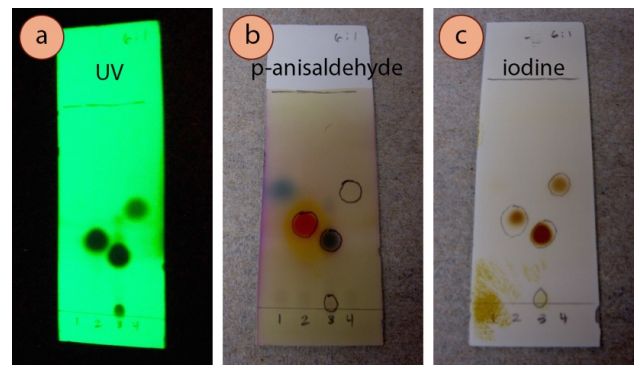
A TLC of 4 samples visualized with three different techniques is shown in Figure ii.36. The plate is visualized with UV light (Effigy two.36b), p-anisaldehyde stain (Figure 2.36c), and vanillin stain (Figure 2.36d). 4-heptanone (lane #i) and acetophenone (lane #2) showed like colorations using the ii stains. Ethyl benzoate (lane #4) was unreactive to both. Cinnamaldehyde (lane #three) was reactive to p-anisaldehyde but not vanillin, while its impurity (cinnamic acid, on the baseline of lane #3) showed the opposite beliefs.
Recipe (p-Anisaldehyde): \(135 \: \text{mL}\) absolute ethanol, \(5 \: \text{mL}\) concentrated \(\ce{H_2SO_4}\), \(1.five \: \text{mL}\) glacial acerb acid, and \(3.7 \: \text{mL}\) p-anisaldehyde. This stain is susceptible to deposition by light, so store wrapped in aluminum foil (Figure two.37e), ideally in the fridge when not in use. Compared to other stains, this stain has a somewhat curt shelf life (approximately half a year). The stain will at first be colorless (Figure 2.37a), merely over time volition turn to a light and so dark pink (Effigy 2.37b-d). The stain is less potent when it darkens, but is oft still usable. Condom notation: wearable gloves while using this highly acidic stain.
Recipe (Vanillin): \(250 \: \text{mL}\) ethanol, \(xv \: \text{g}\) vanillin, and \(2.5 \: \text{mL}\) concentrated \(\ce{H_2SO_4}\). This stain is lite sensitive and should be stored wrapped in aluminum foil in the refrigerator. It is originally light yellow, but darkens over time (Figure two.37f+k). It should exist discarded if it acquires a bluish colour. Safety annotation: wear gloves while using this highly acidic stain.

Reaction Pathways
The p-anisaldehyde and vanillin stains react in a similar style, and normally undergo Aldol and acetalization reactions to produce highly conjugated (and thus colored) compounds on TLC plates.
Aldol Reactions
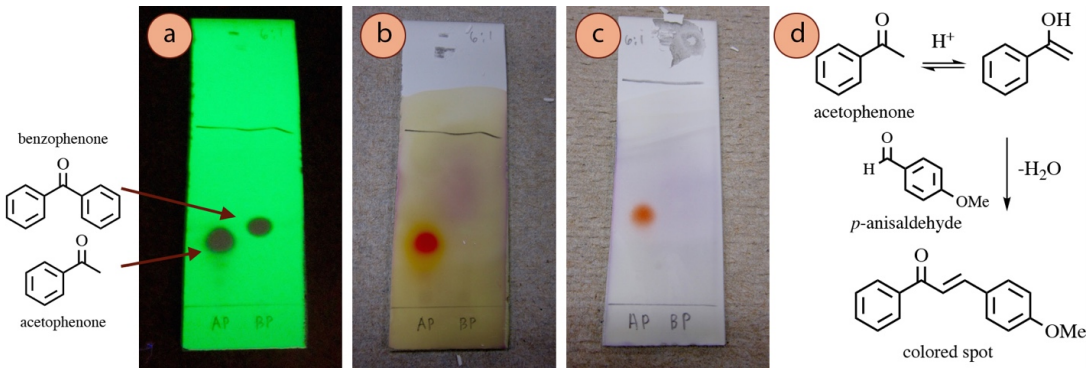
Under the acidic conditions of the stain, some aldehydes or ketones tin can undergo a keto-enol tautomerism, and the enol can undergo acid-catalyzed nucleophilic addition to p-anisaldehyde or vanillin through an aldol mechanism. Dehydration of the aldol product (encouraged past heating the TLC plate), results in a highly-conjugated compound (Figure 2.38d), which is why spots become colored.
For example, a TLC plate containing acetophenone and benzophenone (as seen with UV, Figure 2.38a), are stained with p-anisaldehyde and vanillin stains. Acetophenone produced a colored spot with these stains (Figures 2.38b+c) while benzophenone did not. The main difference is that benzophenone cannot form an enol, or be a nucleophile to p-anisaldehyde, so the stain is unreactive.
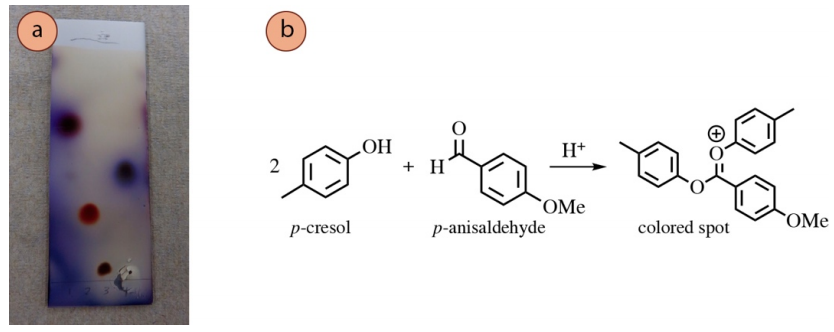
Acetalization Reactions
Some alcohols react with p-anisaldehyde and vanillin stains through acetalization reactions. A proposed reaction of p-cresol with p-anisaldehyde is shown in Effigy 2.39b to produce a highly-conjugated cation, a possible construction of the pink spot on the TLC plate in lane #2 of Figure 2.39a. This cationic structure may look unusual, just is a feasible construction in the highly acidic conditions of the stain.
Permanganate Stain
The permanganate ion \(\left( \ce{MnO_4^-} \right)\) is a deep purple color, and when information technology reacts with compounds on a TLC plate (and is consumed), the plate is left with a xanthous colour (Figure 2.40a). The stain hands visualizes alkenes and alkynes by undergoing improver reactions (Figure two.40d), and the color change is often firsthand with these functional groups.
Permanganate is likewise capable of oxidizing many functional groups (e.k. aldehydes, lane 1 in Figure ii.40c), and then is considered by some to be a universal stain. Heat may exist required to visualize some functional groups, and oftentimes improves the contrast betwixt spots and the groundwork. Heating may be washed (if needed) until the background color just begins to yellow, simply a brown background means the plate was overheated.
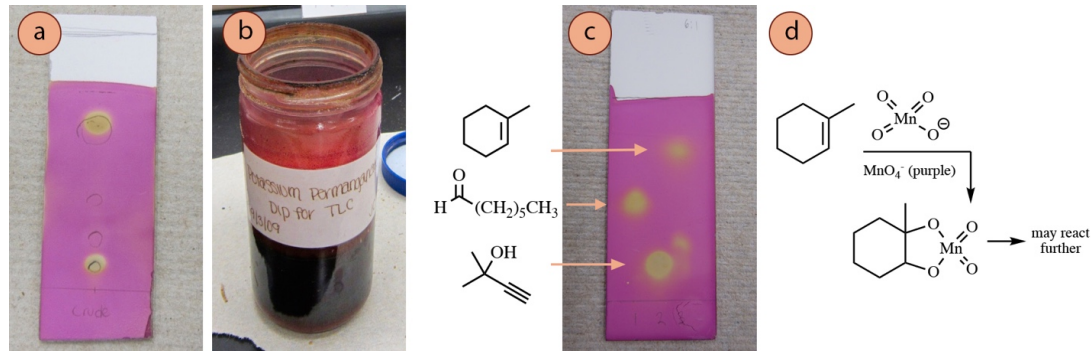
Recipe: \(1.5 \: \text{g}\) \(\ce{KMnO_4}\), \(10 \: \text{g} \: \ce{K_2CO_3}\), \(1.25 \: \text{mL} \: x\% \: \ce{NaOH} \left( aq \correct)\), and \(200 \: \text{mL}\) water. Rubber annotation: wear gloves while using this stain, every bit permanganate is corrosive and will stain skin brownish.
Phosphomolybdic Acid Stain
The phosphomolybdic acid stain (PMA) is considered past some a universal stain, able to visualize a wide variety of compounds (alcohols, alkenes, alkyl iodides, and many carbonyl compounds). The yellowish-green PMA reagent \(\left( \ce{Mo^{half-dozen+}} \correct)\) oxidizes the compound on the plate while itself being reduced to molybdenum bluish (\(\ce{Mo^{5+}}\) or \(\ce{Mo^{4+}}\)). Vigorous heating is required to develop the spots, but the plate is overheated when the groundwork begins to darken. There is typically no color differentiation between spots, as almost compounds visualize as greenish or bluish spots (Figure ii.41c).
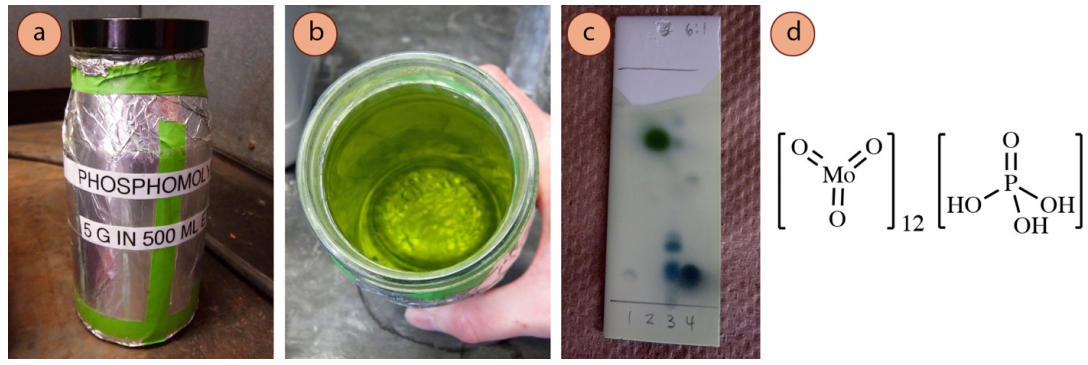
Recipe: \(5 \: \text{1000}\) phosphomolybdic acid in \(500 \: \text{mL}\) ethanol. The stain is light sensitive and and then should exist stored in a jar under aluminum foil. The reagent is expensive, but the stain has a very long shelf life (5+ years).
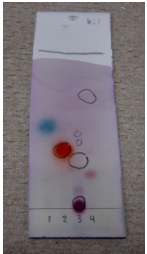
Fe(Iii) Chloride Stain
The ferric chloride \(\left( \ce{FeCl_3} \right)\) stain is highly specific, and is used mainly to visualize phenols \(\left( \ce{ArOH} \right)\). Some carbonyl compounds with loftier enol content may also exist visualized. \(\ce{Fe^{iii+}}\) forms colored complexes with phenols (frequently faint blue), in the full general sense of what is shown in Figure 2.42c. The bodily structure of these complexes is debated\(^vi\). The coloration fades rather rapidly with this stain, and then observations should exist recorded immediately.
.png?revision=1&size=bestfit&width=703&height=317)
Recipe: \(1\%\) \(\ce{FeCl_3}\) in water and \(\ce{CH_3OH}\) (\(50\%\) each). This stain has a high shelf life (v+ years).
Bromocresol Green Stain
The bromocresol dark-green stain is specific for acidic compounds, and should exist able to visualize compounds that produce a solution lower than pH 5. Feel has shown that carboxylic acids piece of work moderately well (beginning lane in Figure 2.43d) but phenols are only barely visible (indicated with an arrow in Effigy 2.43d). In theory, the plate does not need to be heated subsequently exposure to this stain, but in do it often improves the dissimilarity betwixt the spots and the background.
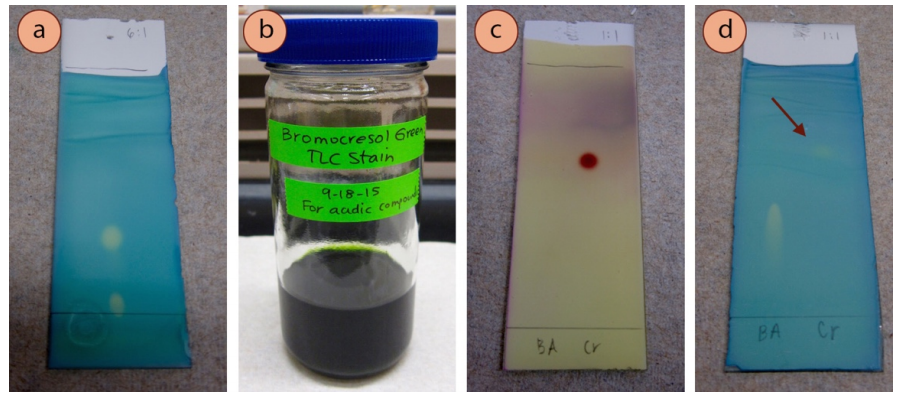
Recipe: \(100 \: \text{mL}\) absolute ethanol, \(0.04 \: \text{k}\) bromocresol green, and \(0.10 \: \text{G} \: \ce{NaOH} \left( aq \correct)\) drop wise until solution turns from yellow to blue (green works besides, as in Figure 2.43b).
This stain uses an acid-base indicator, which works in a like manner to phenolphthalein. Bromocresol light-green is yellow below pH iii.8 and blueish above pH 5.4 (Figure 2.44a). When an acidic chemical compound is spotted on the plate, the acid lowers the pH and causes the indicator to shift to the lower pH yellow form (Figure two.44b).

Visualization Troubleshooting
The Chemical compound Didn't Bear witness Up
Even when a compound has certainly been applied on the baseline of a TLC plate, it is possible that the compound is not seen on the plate after elution. There are several possible reasons for this:
one. The compound may be as well dilute.
A simple solution to a dilution problem is to add more compound to the original sample and run the TLC once more using a new plate. If the chemical compound is expected to be UV active (i.e. if it contains an aromatic ring), it is a adept idea to view the TLC plate under UV lite before eluting the plate (Figure two.45a). If the sample spot is non visible before elution it will not be visible subsequently, as compounds diffuse during elution.
If the sample is determined to be but slightly besides dilute, the material can be deposited multiple times before elution (Figure 2.45b). To do this, deliver a small spot of sample on the baseline, and allow it fully dry out (it helps to accident on it) before delivering another spot over top of the first. If the spots are non immune to dry in between applications, the spot will be too big. If the compound is expected to exist UV agile, bank check the plate under UV light, and if necessary spot more times earlier elution.
ii. The chemical compound may take evaporated.
A TLC plate should exist visualized immediately after elution, so if a moderate amount of time was left between running the TLC and visualizing it, evaporation may be the cause of the problem. A solution to this problem is to run the TLC over again and visualize it immediately.
If the compound has a low humid point, it probably evaporated during elution. For example, ii-pentene (boiling signal \(36^\text{o} \text{C}\)) was spotted in lane #1 of Figure 1.45c. It did not stain with permanganate afterwards elution even though the compound is reactive to the stain (an undiluted, uneluted sample of two-pentene did stain somewhat on a scrap TLC plate, Figure two.45d). Compounds with boiling points lower than approximately \(120^\text{o} \text{C}\) are hard to analyze through TLC.
3. The compound may exist unreactive to the visualization technique.
Visualization techniques are oft tailored toward certain functional groups. For example, ultraviolet light is generally good at visualizing aromatic compounds but poor at other functional groups. If UV, iodine, or a stain fails to visualize a compound, it could mean the compound is simply not reactive to the technique, and some other method should be tried.
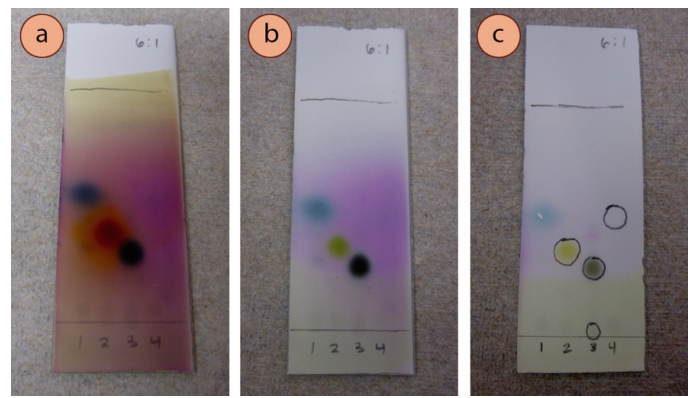
For instance, Figure ii.46 shows for unlike compounds visualized with UV (Figure 2.46a), p-anisaldehyde stain (Effigy two.46b) and iodine (Figure ii.46c). The chemical compound in lane #1 of all the plates (4-heptanone) was only visible with anisaldehyde stain (blue spot), and not with UV or \(\ce{I_2}\). The compound in lane #4 of all the plates (ethyl benzoate) was unreactive to anisaldehyde stain, but could be visualized with UV and \(\ce{I_2}\). The impurity nowadays on the baseline of lane #iii (the impurity cinnamic acid) was strongly UV active, simply could hardly be seen with the other stains.
The Pencil Mark from UV is Different Than the Stain
Ultraviolet light is ofttimes the first visualization technique attempted on an eluted TLC plate because it is nondestructive and rather simple to carry out. If a nighttime spot is seen with a UV lamp, information technology is customary to circumvolve the spot with pencil (as in Figure 2.46b), as the spot will be invisible when the lamp is removed. Some other visualization technique is often carried out later viewing the plate under UV, and it is not uncommon that the subsequent stain extends to a smaller or larger region than the pencil marking.
For example, the compound in lane ii of Figure 2.46 (acetophenone) can exist easily seen with ultraviolet light (Figure two.46a), merely on the plate visualized with iodine (Figure 2.46c), the pencil markings encapsulate a larger region than is seen darkened by the iodine. This is because acetophenone is very strongly UV active, but only mildly complexes with iodine. It is non uncommon for 1 technique to visualize a compound more than finer than some other technique.
It is therefore of import to be cautious in using TLC to interpret the quantity of fabric present in a sample, for example when assessing the quantity of an impurity (such as in lane #iii of Effigy two.46, which contains cinnamaldehyde and its impurity cinnamic acid). Information technology is probable that a large spot is nowadays in a greater quantity than a small spot, but it could also be that the large spot is more responsive to the visualization technique.
A Stain's Colour Faded or Changed With Time
It is very common for the coloration produced by a stain to fade with time, as the compounds somewhen evaporate from the plate or other slower reactions take place. For this reason, it may be a adept thought to circle the spots with pencil immediately after a plate is visualized, although equally spots are more often than not circled after viewing with UV, additional markings may cause confusion as to which compounds are UV-active. Another alternative is to identify articulate tape across the plate to foreclose the spots from evaporating.
It is possible that the coloration produced by a stain will alter with extended heating, or with fourth dimension. For example, the plate in Effigy 2.47 was visualized with p-anisaldehyde stain, and Figure 2.47a shows how the plate appeared immediately after heating. Effigy 2.47b shows how the same plate appeared later on sitting at room temperature for xxx minutes. The compound in lane #2 (acetophenone) had the most dramatic change in color during that time, changing from a bright orange to a green color. Observations recorded into a lab notebook should be of the original color of a spot.
The Staining Doesn't Make Sense
Certain visualization methods work all-time for certain functional groups, so a positive result with a stain tin can give clues nearly the identity of an unknown spot. However, sometimes a compound stains when information technology isn't "supposed to", and this can be confusing.
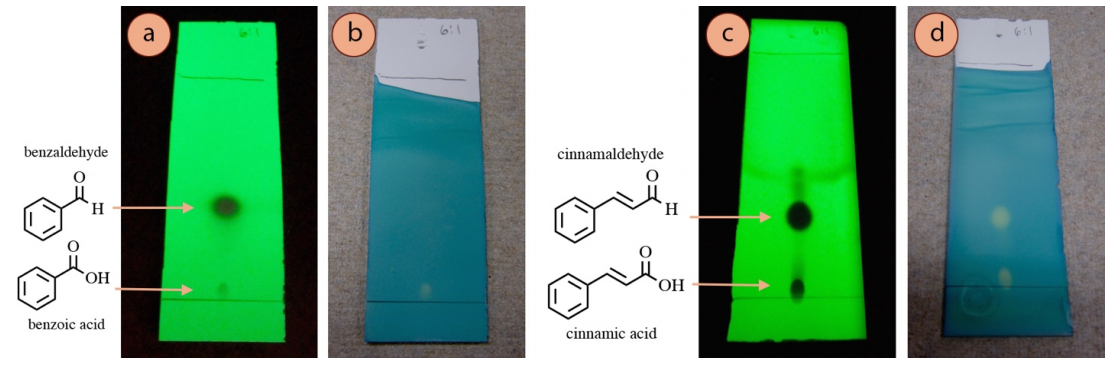
For example, a TLC of benzaldehyde visualized with UV low-cal (Figure 2.48a) shows two spots, and based on relative \(R_f\) values, it would make sense that the dark spot is benzaldehyde and the fainter spot virtually the baseline is benzoic acid (acquired by an oxidation of benzaldehyde). Staining of the plate with bromocresol green (a stain for acidic compounds), supports this hypothesis as the lower acidic spot is visualized with this method (Figure 2.48b). This is an instance of when the staining results "make structural sense", and can even support the identification of unknown spots.
However, in a similar experiment with cinnamaldehyde, both aldehyde and carboxylic acid spots were strongly visualized with bromocresol green (Effigy ii.48d), even though only one is an acidic compound. This consequence does non at first "make sense", and theories can only be postulated for why the aldehyde reacted with the stain.
The significance of Effigy ii.48 is that interpretation of staining or lack of staining can sometimes exist used to infer structure, but there may be exceptions that are difficult to explain.
\(^six\)Meet Nature 165, 1012 (24 June 1950); DOI: 10.1038/1651012b0
\(^7\)The TLC plate was left in the \(\ce{I_2}\) sleeping accommodation for merely near 2 minutes, and the spots may take developed further with additional fourth dimension.
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_Lab_Techniques_(Nichols)/02%3A_Chromatography/2.03%3A_Thin_Layer_Chromatography_(TLC)/2.3F%3A_Visualizing_TLC_Plates
0 Response to "what is true about fecl3 that allows it to form a colored complex with phenols?"
Postar um comentário